All perceptions (e.g., hot, cold, loud, bright, smelly, hard, soft, sour, sweet, pain, etc.) are cortical, meaning perceived in the brain. All pain is perceived in the brain (1).
Pain perception is brought to the brain by nerves. Structures that do not have a nerve supply (like articular hyaline cartilage, fingernail) cannot send the pain signal to the brain. All structures that do have a nerve supply can initiate a pain signal and send it to the brain.
The pain signal is an electrical phenomenon. As an analogy, the pain “wire” for the electrical signal is the neuron.
The pain “wire” (neuron) has specific parts that are important to this discussion:
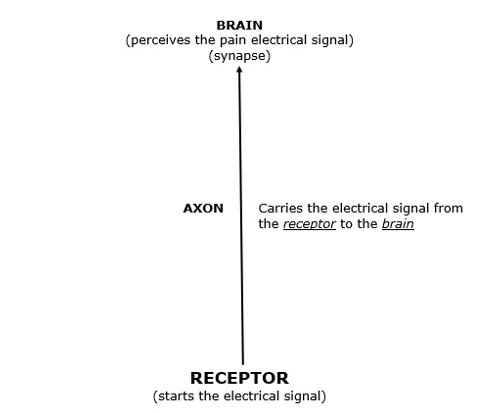
The Pain Receptor
The receptor is found at the end of the sensory neuron. This includes soft tissues (ligament, muscles, skin, fascia, intervertebral disc, etc.). It also includes hard tissues (bone).
The receptor has the ability to take an environmental stress and convert it to an electrical signal.
Soft tissue injuries, inflammations, and/or irritations can initiate the pain electrical signal at the receptor, and the receptor will propagate the electrical signal along the axon to the brain where the signal is perceived.
The Axon
As noted above, the primary responsibility of the axon is to propagate the electrical signal from the receptor to the brain.
There is an important exception to this, which will be explained later in this publication.
The Brain
Also, as noted above, it is the brain that perceives the pain electrical signal that is delivered to it by the axon.
Soft Tissue Injury and Repair
When watching sports, like the Super Bowl, one will observe numerous injuries. The sportscasters will often tell their audience the reason why a number of a team’s top players are not in the game, detailing the injuries that the absent player is recovering from. It is rare for these injuries to be fractures, or what is referred to as hard tissue injury. The majority of these injuries are considered to be a soft tissue injury.
Soft tissues include ligaments, muscles, skin, fascia, intervertebral disc, etc. Essentially, all injuries that are not to the bone are considered to be a soft tissue injury. Technically, an injury to the nerve itself is a soft tissue injury. However, important for this discussion, an injury to the nerve itself will not be considered to be a soft tissue injury. This discussion will consider a nerve injury to be in a separate category of injury called neuropathic injury.
Injured soft tissues heal in three distinct phases (2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12):
- Phase 1: the acute inflammatory phase
- Phase 2: the repair or proliferation phase
- Phase 3: remodeling phase
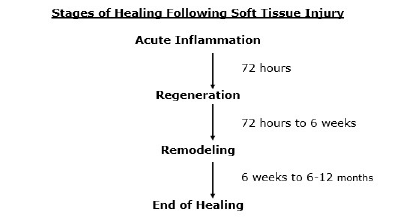
Phase 1: The Acute Inflammatory or Reaction Phase
This phase of healing lasts up to about 72 hours. It is characterized by vasodilation, immune system activation of phagocytosis by macrophages to remove debris, and the release of prostaglandins and other inflammatory molecules.
The inflammatory chemicals play a prominent role in pain production, the increase in capillary permeability, and swelling.
The wound is hypoxic because the blood vessels have been disrupted, but immune system macrophages perform their phagocytosis duties anaerobically.
Phase 2: The Repair or Regeneration Phase
This phase begins at about 48 hours and continues for approximately 6 to 8 weeks. This phase is characterized by the synthesis and deposition of collagen, which literally glues the margins of the healing breach together.
The collagen that is deposited in this phase is not fully oriented in the direction of tensile strength. Rather, it is laid down in an irregular, non-physiological pattern.
Phase 3: The Remodeling Phase
This phase may last up to “12 months or more” (12). The remodeling phase is mostly influenced and controlled through the use of controlled motion. This is an important clinical application for chiropractors who use controlled motion as a benefit of joint manipulation (specific and controlled line-of-drive motion). This point is emphasized in the following publications:
••••
(6)
“Early mobilization, guided by the pain response, promotes a more rapid return to full activity.”
“Early mobilization, guided by the pain response, promotes a more rapid return to full functional recovery.”
“Following this acute inflammatory phase and largely guided by the pain response of the patient, early mobilization is commenced, based upon the premise that the stress of movement on repairing collagen is largely responsible for the orientation and tensile strength of the tendons and ligaments.”
“The goal of stressing repairing tissues with controlled motion is to induce adaptive response of functionally stronger connective tissues.”
“Collagen fiber growth and realignment can be stimulated by early tensile loading of muscle, tendon, and ligament.”
••••
(10)
“The large scar tissue mass gradually remodels, likely under the influence of the mechanical environment.”
“Maturation of the scar tissue requires mechanical loading to continue the remodeling phase of healing.”
“Normal connective tissues that function in a mechanically active environment (actually most tissues) subscribe to the ‘use it or lose it’ paradigm of tissue integrity.”
“Increased loading leads to adaptation, whereas decreased loading below a threshold leads to atrophy.”
“Mechanobiology is likely important in the healing outcome in tissues such as ligaments, tendons, and related tissues. That is, depriving healing ligaments of mechanical loading likely has a detrimental impact on healing outcome.”
••••
(11)
“For [the] collagen network to attain an almost identical construction of the original tissue, the tissue in this phase of wound healing must be confronted with its normal physiological stress.”
“An important task for the therapist is to apply gradually increasing levels of force without causing pain, in order to promote the healing and regeneration processes and in this way restore mobility and stability.”
Most healed soft tissue injuries are asymptomatic. However, it is universally accepted that the healed tissue is weaker than the pre-injured tissue. Consequently, acute flare-ups of pain or exacerbations of pain and/or spasm often occur as a consequence of increased use or stress of the once injured but now healed tissues. Good early treatment improves the quality and timing of soft tissue injury. Best early treatment appears to include ice and early controlled motion. This would include chiropractic care involving adjusting of the injured joints.
•••••••••
Problem Solving
A common and legitimate question is “Why do some soft tissue injuries take a prolonged period of time to heal?” It is a reality: some patients do not recover as expected and/or take a longer period of time to become maximumly improved. This question is asked by all parties involved: the patient, doctors, chiropractors, insurance companies, claims adjusters, health plan administrators, lawyers, courts, family and friends, etc. The answer may be right in front of all involved. It has been described in the scientific literature for decades:
Neuropathic Pain Syndrome
The prior discussion pertained to soft tissue injuries that cause inflammations and/or irritations to the nerve receptors. These nerve receptors reside within those tissues. As mentioned above, technically, neuropathic pain is a soft tissue injury. But in contrast to other soft tissue injuries, the consequences and prognosis for neuropathic injury are far more difficult and less optimistic.
Simply stated, neuropathic pain is pain caused from injury, damage, or dysfunction of the nerve itself. Neuropathic pain is distinct from the pain caused by soft tissue injury. Neuropathic pain tends to become chronic and debilitating. “Neuropathic pain affects approximately 3–17% of the chronic pain population in the world” (13).
There are several Clinical Questionnaires to help establish the presence and clinical outcomes for neuropathic pain. An example of a popular one, the DN-4, is included on page 10 of this publication.
Most musculoskeletal practitioners are unfamiliar with the concept of neuropathic pain. Yet, a search of the U.S. National Library of Medicine, using the PUBMED search engine and the words “neuropathic pain,” identifies 56,609 citations (as of February 6, 2024). The oldest of these publications appeared in the 1930s. In 2015, the International Association for the Study of Pain (IASP) declared that year to be the “global year against neuropathic pain” (14).
Typical neuropathic pain categories include:
- Lumbosacral radiculopathy
- Piriformis sciatica
- Carpal tunnel syndrome
- Cervical radiculopathy
- Neurogenic thoracic outlet syndrome
- Chemotherapy-induced peripheral neuropathy
- Spinal cord injury
- Diabetic polyneuropathy
- Post stroke pain
- Chronic inflammatory demyelinating polyneuropathy
- Entrapment neuropathy
- Trigeminal neuralgia
Several of these are rarely or never seen in chiropractic clinical practice (chemotherapy-induced peripheral neuropathy, spinal cord injury, diabetic polyneuropathy, post stroke pain, demyelinating polyneuropathy).
In contrast, some of these are quite common in chiropractic clinical practice and often successfully resolved or acceptably improved with chiropractic care, including spinal adjusting (lumbosacral radiculopathy, piriformis sciatica, cervical radiculopathy, neurogenic thoracic outlet syndrome, carpal tunnel syndrome, entrapment neuropathy, trigeminal neuralgia). The radiculopathy neuropathic pain syndromes are particularly relevant to the chiropractic community because the nerve roots exit between the spinal vertebrae. Disc pathology, facet injury, uncinate joint injury, and spinal arthrosis and/or spondylosis all have the ability to injure, inflame, and/or irritate the adjacent nerve root axions.
A subjective hallmark of neuropathic pain has the patient complaining of multiple characteristics. These might include burning, painful cold, electric shocks, tingling, pins and needles, numbness, itching, etc. No two patients are exactly alike. Each patient will present uniquely different.
The clinical assessment of neuropathic pain syndrome is not a simple task. Credible studies on the topic of neuropathic pain syndrome often use similar but often somewhat different questionnaires to determine the presence of neuropathic pain syndrome (15, 16). A representative example of a commonly used questionnaire is attached at the end of this publication.
In 1958, the Journal of the American Medical Association published a study titled (17):
Whiplash Injuries:
Neurophysiological Basis for Pain and Methods Used for Rehabilitation
The author, Beverly Hills neurosurgeon Emil Seletz, MD, states:
“The person’s body (in the car that is struck) continues to move forward, while the head, being hinged at the neck, snaps backwards. The average head weighs about 8 lbs., and the cervical vertebrae are very delicate; the force that is pushing the head backwards is even greater than believed, since the base of the neck acts as a fulcrum and the leverage is applied near the top of the head.”
“Therefore, the head snaps back with the equivalent of several tons of force—without any support, since ‘the muscular control of the neck is caught off guard.’”
“The end-result, with the neck in acute hyperextension, is a momentary posterior subluxation of the various joints with fleeting narrowing of the foramina, so that the nerve root is caught in a pinchers between the superior and inferior facets.”
This discussion, “the nerve root is caught in a pinchers between the superior and inferior facets,” presents a plausible argument for the etiology of neuropathic pain from whiplash mechanism injuries.
A recent publication (2022) in the journal Pain quantified the incidence of neuropathic pain following whiplash collisions in a study titled (18):
Nerve Pathology and Neuropathic Pain After Whiplash Injury:
A Systematic Review and Meta-Analysis
This study is quite large. It reviewed 54 studies reporting on 390,644 patients and 918 controls.
The authors note that about 50% of patients suffering from whiplash injuries will suffer from chronic pain. They note that an explanation for this high rate of chronicity is that the patients are suffering not from soft-tissue injury alone, but rather from neuropathic pain syndrome.
The authors note that the prevalence of neuropathic pain in these whiplash-injured subjects was 34% to 75%. They state:
“There is no clear understanding of the mechanisms causing persistent pain in patients with whiplash associated disorder (WAD).”
“There is increasing evidence of nerve involvement and neuropathic pain in patients with chronic WAD.”
“Our systematic review including 54 studies in 390,644 patients suggests that after whiplash injury, a subset of people demonstrate signs of peripheral nerve injury and/or neuropathic pain.”
“Neuropathic pain is reported by a significant group of patients with WAD.”
“Our data suggest that nerve pathology and signs of neuropathic pain are present in a subset of patients after whiplash injury.”
The DN-4 Questionnaire Estimates the Probability of Neuropathic Pain
This questionnaire has been well validated in a number of studies and is considered to be one of the most suitable neuropathic pain screening tools for clinical use:
7 symptom items are scored by interviewing the patient.
3 items are scored by means of clinical examination.
The scores are added and a score of 4 or more out of 10 is suggestive of neuropathic pain. YES = 1 Point; NO = 0 Points; Patient’s Score_______
Interviewing the Patient
QUESTION 1: Does the pain have one or more of the following characteristics?
| Burning | YES | NO |
| Painful Cold | YES | NO |
| Electric shocks | YES | NO |
QUESTION 2: Is the pain associated with one or more of the following symptoms in the same area?
| Tingling | YES | NO |
| Pins and needles | YES | NO |
| Numbness | YES | NO |
| Itching | YES | NO |
Examination of the Patient
QUESTION 3: Is the pain located in an area where the physical examination may reveal one or more of the following characteristics?
| Hypoesthesia to touch | YES | NO |
| Hypoesthesia to pinprick | YES | NO |
QUESTION 4: In the painful area, can the pain be caused or increased by?
| Brushing | YES | NO |
Future Directions and Concepts
Cytokines are protein molecules that are produced by immune system cells. Cytokines can be pro-inflammatory or anti-inflammatory.
Interleukins are a category of cytokines. Like cytokines, interleukins can be either be pro-inflammatory or anti-inflammatory. Interleukins are abbreviated “IL.”
IL-27 and IL-10 are both anti-inflammatory cytokines. An important article pertaining to neuropathic pain was published in the journal Frontiers in Immunology in 2020, titled (19):
IL-27 Counteracts Neuropathic Pain Development
Through Induction of IL-10
These authors propose that, ideally, following axonal nerve injury, the immune system cells will increase production of anti-inflammatory cytokine IL-27. In turn, IL-27 will induce an increase in the anti-nociceptive cytokine IL-10, which inhibits neuropathic pain development following injury. The authors state:
“These results provided evidence that IL-27 is a cytokine produced after peripheral nerve injury that counteracts neuropathic pain development through induction of the antinociceptive cytokine IL-10.”
The authors conclude that interventions that increase IL-27 and IL-10 “could emerge as possible therapeutic approaches for the prevention of neuropathic pain development after peripheral nerve injury.”
This science and perspective has a lot of relevance for the chiropractic profession and for their patients suffering from neuropathic pain. In 2016, the World Federation of Chiropractic award winning paper was published in the Journal of Manipulative and Physiological Therapeutics, titled (20):
Attenuation Effect of Spinal Manipulation on Neuropathic and Postoperative Pain Through Activating Endogenous Anti-Inflammatory Cytokine Interleukin-10 in Rat Spinal Cord
Using animal models, these authors showed that repetitive spinal manipulative therapy “significantly reduced simulated neuropathic and postoperative pain, inhibited or reversed the neurochemical alterations, and increased the anti-inflammatory IL-10 in the spinal cord.” They concluded:
“These findings show that spinal manipulation may activate the endogenous anti-inflammatory cytokine IL-10 in the spinal cord and thus has the potential to alleviate neuropathic and postoperative pain.”
••••
The benefits of chiropractic care, including spinal adjusting, are unquestioned in the management of neuropathic pain syndromes, especially for spinal radiculopathy syndromes. Perhaps, this elevation of IL-10 following spinal adjusting is a plausible explanation.
REFERENCES
- Ambron R; The Brain and Pain: Breakthroughs in Neuroscience; Columbia University Press; New York; 2022.
- Adams J, Peng W, Cramer H, Sundberg T, Moore C; The Prevalence, Patterns, and Predictors of Chiropractic Use Among US Adults; Results From the 2012 National Health Interview Survey; Spine; December 1, 2017; Vol. 42; No. 23; pp. 1810–1816.
- Oakes BW; Acute Soft Tissue Injuries; Australian Family Physician; 1982; Vol. 10; No. 7; pp. 3-16.
- Roy S, Irvin R; Sports Medicine: Prevention, Evaluation, Management, and Rehabilitation; Prentice-Hall, Inc.; 1983.
- Frank C, Amiel D, Woo S, Akeson W; Normal Ligament Properties and Ligament Healing; Clinical Orthopedics and Related Research; June 1985.
- Kellett J; Acute Soft Tissue Injuries: A Review of the Literature; Medicine and Science of Sports and Exercise; American College of Sports Medicine; 1985; Vol. 18; No. 5; pp. 489-500.
- Woo S (ed.); Injury and Repair of the Musculoskeletal Soft Tissues; American Academy of Orthopaedic Surgeons; 1988.
- Cohen IK, Diegelmann RF, Lindbald WJ; Wound Healing, Biochemical & Clinical Aspects; WB Saunders, 1992.
- Kannus P; Immobilization or Early Mobilization After an Acute Soft-Tissue Injury?; The Physician And Sports Medicine; March 2000; Vol. 26; No. 3; pp. 55-63.
- Hildebrand KA, Gallant-Behm CL, Kydd AS, Hart DA; The Basics of Soft Tissue Healing and General Factors that Influence Such Healing; Sports Medicine Arthroscopic Review; September 2005; Vol. 13; No. 3; pp. 136–144.
- Schleip R; Fascia: The Tensional Network of the Human Body; The Scientific and Clinical Applications in Manual and Movement Therapy; Churchill Livingstone; 2012.
- Hauser RA, Dolan EE, Phillips HJ, Newlin AC, Moore RE, Woldin BA; Ligament Injury and Healing: A Review of Current Clinical Diagnostics and Therapeutics; The Open Rehabilitation Journal; 2013; No. 6; pp. 1-20.
- van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N; Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies; Pain; April 2014; Vol. 155; No. 4; pp. 654–662
- IASP; Global year against neuropathic pain. 2015; Available at: http://www.iasp-pain.org/GlobalYear/NeuropathicPain?navItemNumber5580.
- Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice ASC, Serra J, Smith BH, Treede RD, Jensen TS; Neuropathic Pain: An Updated Grading System for Research and Clinical Practice; Pain August 2016; Vol. 157; No. 8; pp. 1599–1606.
- Madani SP, Abdolmaleki K, Ahadi T, Mansoori K, Raissi GR; Neuropathic Pain Symptom Inventory (NPSI) Questionnaire-Persian Version Can Differentiate Neuropathic from Non-Neuropathic Pain; Pain Management Nursing; February 2023; Vol. 24; No. 1; pp. 96-101.
- Seletz E; Whiplash Injuries: Neurophysiological Basis for Pain and Methods Used for Rehabilitation; Journal of the American Medical Association; November 29, 1958; Vol. 168; No. 13; pp. 1750-1755.
- Fundaun J, Kolski M, Baskozos G, Dilley A, Sterling M, Schmid AB; Nerve Pathology and Neuropathic Pain After Whiplash Injury: A Systematic Review and Meta-Analysis; Pain; July 1, 2022; Vol. 163; No. 7; pp. e789-e811
- Fonseca MM, Davoli-Ferreira M, Santa-Cecília F, Guimarães RM, Oliveira FFB, Kusuda R, Ferreira DW, Alves-Filho JC, Cunha FQ, Cunha TM; IL-27 Counteracts Neuropathic Pain Development Through Induction of IL-10; Frontiers in Immunology; January 28, 2020; Vol. 10; Article 3059.
- Song XJ, Huang ZJ, Song WB, Song XS, Fuhr AF, Rosner AL, Ndtan H, Rupert RL; Attenuation Effect of Spinal Manipulation on Neuropathic and Postoperative Pain Through Activating Endogenous Anti-Inflammatory Cytokine Interleukin-10 in Rat Spinal Cord; Journal of Manipulative and Physiological Therapeutics; January 2016; Vol. 39; No. 1; pp. 42-53.
“Authored by Dan Murphy, D.C.. Published by ChiroTrust® – This publication is not meant to offer treatment advice or protocols. Cited material is not necessarily the opinion of the author or publisher.”



